A mixture of a weak acid and its conjugate base or a mixture of a weak base and its conjugate acid is called a buffer solution or a buffer. It reacts with metals produces H 2.

Predicting Products Of Acid Base Reactions Youtube
12 Factors affecting acidic strength.
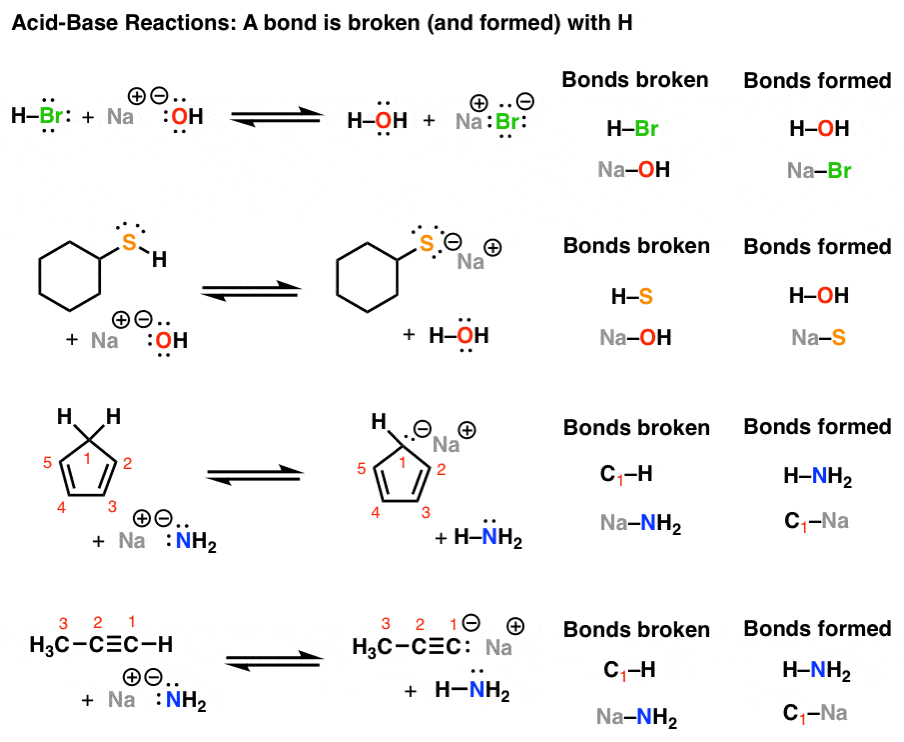
. Historically this reaction was represented as acid base alkali salt water. The strength of acids and bases depends on following factors. For example the reaction between sodium hydroxide base and hydrochloric acid forms sodium chloride salt and water.
The statement is still valid as long as it is understood that in an aqueous solution the substances involved are subject to dissociation which changes the ionization state of the substances. Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added Figure PageIndex1. HCl NaOH NaCl H 2 O.
The arrow sign is used because the reaction is complete that is. The products are less acidic or basic than reactants. Polarity of the molecule and strength of H A bond.
A solution of acetic acid ceCH3COOH and sodium acetate ceCH3COONa is an example of a.

Acid Base Reactions In Organic Chemistry Master Organic Chemistry

Introduction To Acid Base Reactions Master Organic Chemistry
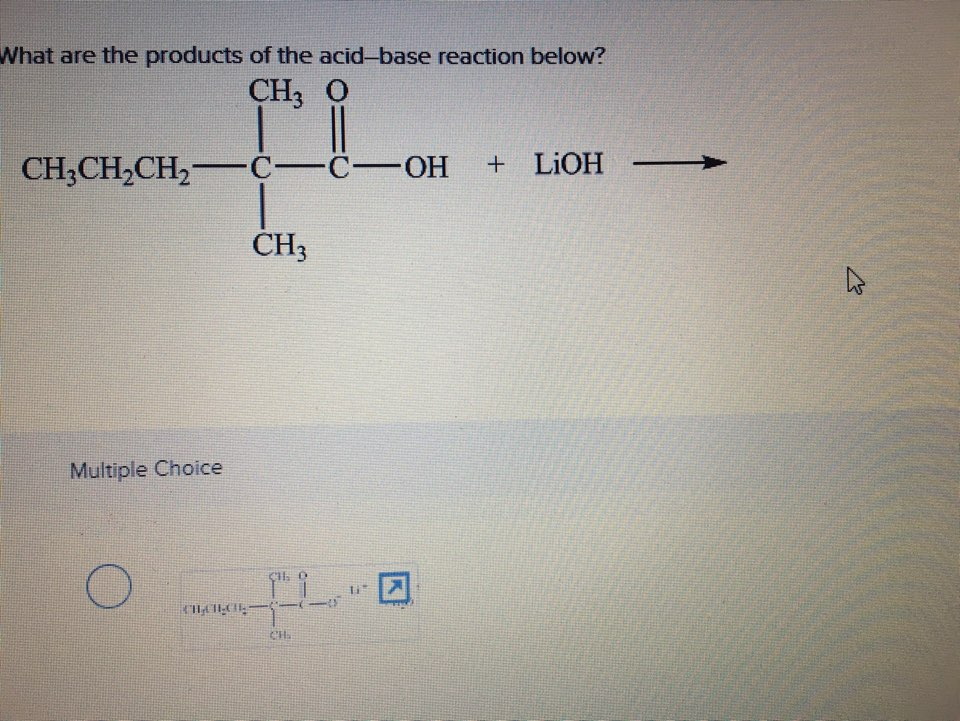
Solved What Are The Products Of The Acid Base Reaction Chegg Com
0 Comments